Manjurul/Getty Images

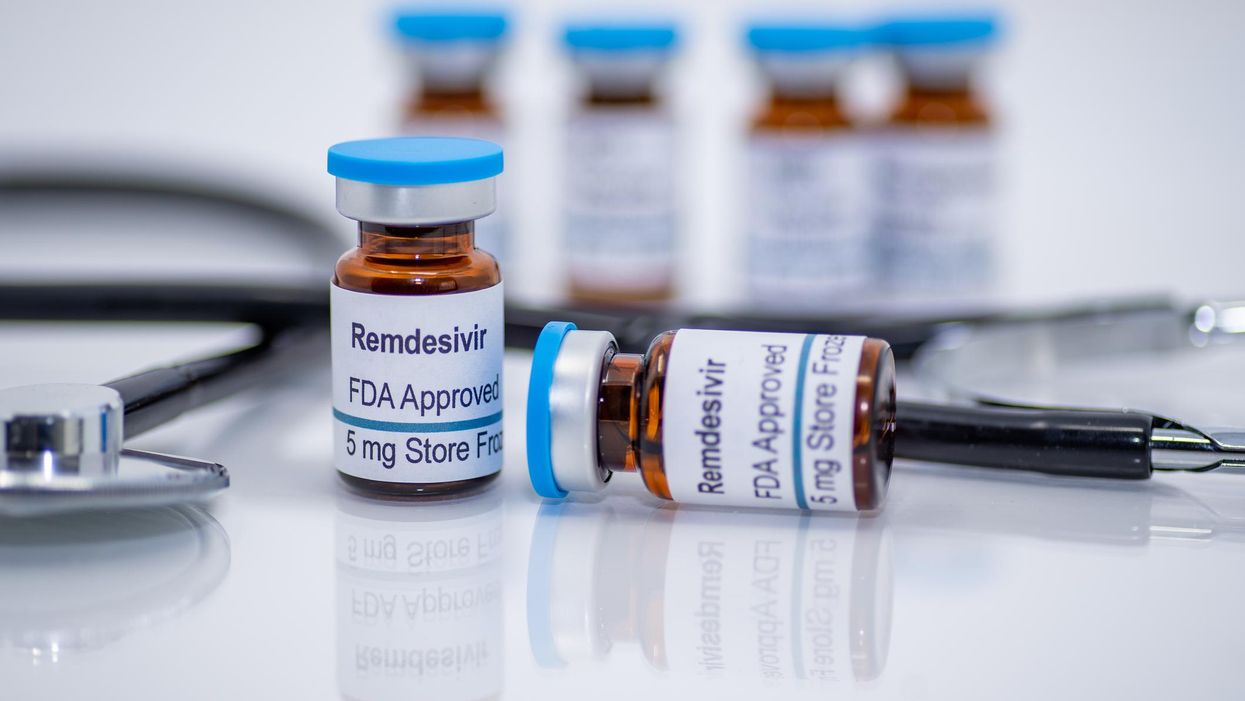
Why in the world would our government reject nearly every cheap, safe repurposed drug with literature showing efficacy against COVID while embracing an expensive drug that was so toxic to the kidneys that it had to be pulled from an Ebola trial? Well, now we have a billion reasons.
There is no doctor in the hospital who will look you in the eye and tell you that remdesivir works. Yet, despite its risk of causing organ failure, our government has refused to suspend its status as standard of care, much less take it off the market, over a year and a half after it was approved and clearly netted no positive results. Why? Follow the money. Modern Healthcare reported last week that hospitals spent $1 billion on Gilead’s remdesivir, more than on any other drug during the pandemic. As the article notes, this is the first time in a decade that AbbVie's rheumatoid arthritis biologic Humira did not top the list for most money spent on a hospital drug.
However, this is not really about the hospitals. It’s about the government paying off the pharma lobbyists who own them. After all, most of the pandemic hospital payments came from some form of government program allocations. According to a document obtained by STAT News, the Biden administration misallocated $7 billion in congressionally earmarked funds intended to help hospital staffing and supply and repurposed the funding to pay off the drug manufacturers for the vaccines and failed therapeutics. In total, $16.7 billion has been siphoned off for the drug companies since the beginning of the pandemic.
Thus, the next time someone asks you why cheap, safe, repurposed drugs with decades-long safety profiles were rejected to accommodate new dangerous drugs, don’t act surprised or attempt to explain the malfeasance using science and medicine. The NIH admits that remdesivir could cause liver toxicity and kidney failure. The WHO recommends against using the drug, and a study published in JAMA showed it increased hospital stays. Now, they are attempting to take their road show to outpatients.
Last week, the FDA announced approval of remdesivir for pediatric use as well as for the elderly for “mild to moderate COVID,” even outpatient. Bizarrely, even for outpatient use, they are still using the intravenous version of the drug, which should raise concerns as to why an oral treatment has not come on the market for the past 18 months. What do they know that we don’t?
Also, remember, unlike ivermectin, which has 20 mechanisms of action spanning through the inflammatory and blood-clotting stages of the disease, remdesivir cannot work once the inflammatory reaction occurs. In what universe of science and medicine would remdesivir become the standard of care for a billion dollars while ivermectin and numerous other common repurposed drugs that cause no serious side effects are essentially criminalized? And let’s never forget that remdesivir was developed by UNC Chapel Hill in the same lab that originally applied for the gain-of-function research on ACE-2 binding of coronavirus spike proteins.
But remdesivir isn’t the only scandalous drug on the block. In fact, the approval and payoff of big pharma have been corrupt and concerning with regard to every COVID drug on the market. Take Merck’s molnupiravir, for example. The drug is potentially mutagenic, carcinogenic, and showed no meaningful efficacy even in early stages of COVID during the second half of the trial. On the printed label for the drug, the FDA issued the following warning on reproductive health:
So, we have zero proof of efficacy, no long-term trials, no cancer studies, but they already know you must use contraception for three months after taking the drug! As CNBC reported last year, molnupiravir was found to be lethal to embryos in pregnant rats, in addition to causing birth defects and reducing fetal body weight. In the dog trials, it also caused birth defects and interfered with bone growth of young pups. Last month, the New York Times reported on a University of North Carolina study that found mutations in hamsters after they were administered the drug. The authors suggest that studies are needed to tell whether these “mutations in host DNA could contribute to the development of cancer, or cause birth defects either in a developing fetus or through incorporation into sperm precursor cells.”
Again, our government is spending billions of dollars on this, while banning cheap, safe drugs. Oh, and molnupiravir was never tested against the current dominant variant. Just like with remdesivir, try defending the government’s approval of molnupiravir juxtaposed to its war against hydroxychloroquine and ivermectin.
What about Pfizer’s Paxlovid? That drug for sure smells like a rose because it was made by Pfizer, and everything the company makes is at least “90%” effective, right? Well, because it’s mixed with AIDS drug ritonavir, it is contraindicated with 32 entire classes of drugs, many of which are universal to those at risk for COVID because of other underlying conditions. It also comes with the following FDA warning:
Think about the gravity of this situation: Why would we ignore a drug like ivermectin that is so safe and has 20 mechanisms of action, to the benefit of an expensive drug that is still being studied, is contraindicated with so many common drugs, and only has one front-end mechanism of action that is unlikely to work beyond the first three days of symptoms?
In a letter to the Lancet, a group of British doctors recently warned that “the use of ritonavir was complicated by high pill burden, poor tolerability, and drug interactions.” Specifically, they point to “interactions that might lead to life-threatening adverse events” in those who take “statins, steroids, sedative hypnotics, anticoagulants, and antiarrhythmic therapies, many of which are prescribed separately in older populations (aged ≥70 years) at the greatest risk of complications from SARS-CoV-2 infection.” Thus, typically, you need multi-drug therapy to deal with a complicated case of COVID, yet we are going to sacrifice those drugs for Pfizer’s drug that couldn’t work after 72 hours?
And let’s not forget, just like with the vaccines, remdesivir, and molnupiravir, there are no trials on its use against Omicron. It’s true that a computer model study did show efficacy of Paxlovid as a protease binder against Omicron, but guess what? That same study concluded, “Ivermectin showed the highest binding affinity and may be the most effective drug candidate against the Omicron variant.” Plus, ivermectin can be used later in the disease because of its anti-inflammatory and anti-coagulant properties.
We now know from a letter written by a DARPA researcher to the DOD inspector general, obtained by Project Veritas, that our government knew early on that ivermectin and hydroxychloroquine have auspicious mechanisms of action against SARS-CoV-2. “Ivermectin (identified as curative in April 2020) works throughout all phases of illness because it both inhibits viral replication and modulates the immune response,” wrote Major Mike Murphy, a DARPA researcher in the now unclassified letter dated Aug. 13, 2021. “Of note, chloroquine phosphate (Hydroxychloroquine, identified April 2020 as curative) is identified in the proposal as a SARSr-CoV inhibitor, as is interferon (identified May 2020 as curative).”
How many lives could have been saved if a fraction of the funding placed into the shots, remdesivir, molnupiravir, and Paxlovid were instead used to research the best combination and dosage of cheap repurposed drugs? That is the billion-dollar question we will never have the answer to without a Nuremberg trial 2.0.Daniel Horowitz
Blaze Podcast Host